
KIRO Oncology
Robotic compounding system for sterile preparations of hazardous drugs
Features
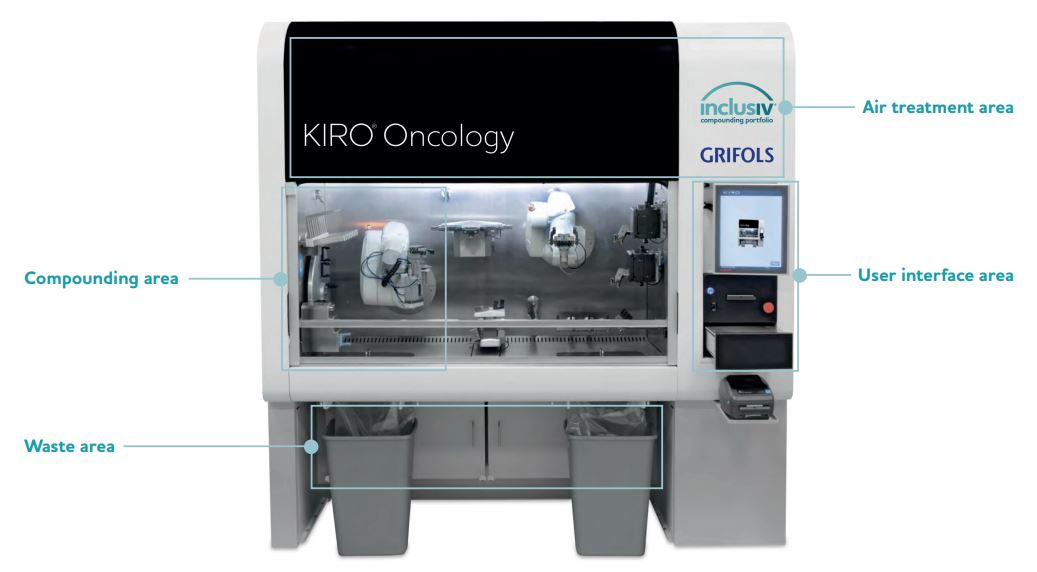
Compounding Area
- Two robotic arms
- 12-position carousel for vials in use (1-100 mL)
- Preparation bay for up to 8 infusion bags, cassettes, or elastomeric pumps
- Syringe holder for up to 8 syringes (1, 3, 10, 20, and 50 mL)
- Syringe capping station with 4 capping positions
- Holding area for up to 10 partially used vials
- Gravimetric device for in process weighing
- Two peristaltic pumps for diluent filling of empty containers and reconstitution of lyophilized drug vials
- Two cameras for syringe and vial identification, respectively
- Barcode reader for product identification
User Interface Area
- User touchscreen interface
- Gravimetric device to double check weighing
- Barcode reader for product identification
- Two label printers (small and large labels)
Air Treatment Area
- ISO 5 Compounding Area
- Environmental protection by high efficiency particulate air (HEPA) exhaust filter and negative pressure control in recirculation and exhaust
- User protection by enclosed compounding environment under negative pressure
- 30% exhaust and 100% exhaust options available
Waste Area
- Two self-enclosed waste disposal units to prevent user exposure
- Two additional bag-in/bag-out filters for air cleaning before recirculation
WATCH THE VIDEO FOR MORE INFORMATION.
KIRO Oncology Key Benefits
Solutions Designed to Keep Your Patients Safe

Patient Safety
- Precision scales for gravimetric verification at all stages of the compounding process to control dosing accuracy
- In-process barcode readers and cameras identify drugs, disposables, and final containers to document traceability
Staff Protection
- Self-cleaning process avoids manual cleaning
- Automated compounding and self-cleaning occur in a completely enclosed environment
- Integrated system for automatic disposal of hazardous waste into self-contained bags
- Operators are protected from repetitive stress injuries


Flexibility and Efficiency
- Supports a wide variety of vials and final containers
- Prepares patient-specific doses and small batches using liquid or lyophilized drugs
- Device and user efficiency increased by allowing identification and labelling of materials by the user during automatic compounding
- Software guided combination, replacement and reuse of vials for optimal drug use including partially used vial tracking and labelling
- Workflow optimized by visual planning board
- Configuration options to respond to specific compounding practices, workflows, and clinical needs
Regulatory Compliance
- Compliance with USP and GMP regulations supported by standardizing aseptic procedures, facilitating personnel and process qualification, and minimizing risk of exposure when preparing hazardous drugs
- Airflow operation and temperature control, and optional continuous particle counter to meet GMP requirements
- Fully serviced during deployment, qualification, and periodic maintenance, including dosing accuracy tests and air flow certifications addressing smoke tests under dynamic conditions for USP <797> compliance
- Syringes can be automatically capped with closed system drug transfer devices (CSTDs) and infusion bags loaded with a pre-attached CSTD spike to supply closed containers and meet USP <800> requirements for the administration of hazardous drugs
- KIRO Oncology meets electrical safety requirements by being UL listing marked per IEC 61010 and EMC certified per IEC 61326-1
Technical information
- Size (w x d x h): 83″ x 45″ x 88″ (2100 mm x 1133 mm x 2235 mm)
- Minimum clearance (w x d x h): 118″ x 98″ x 98″ (3000 mm x 2500 mm x 2500 mm)
- Weight: 1200 Kg (2645 lb)
- Required minimum floor load rating: 200 kg/m² (40.9 lbf/ft²)
- Exhaust airflow rate: 500 m³/h (300 CFM) for 30% exhaust and 2000 m³/h (1200 CFM) for 100% exhaust
- Power: 120 VAC ± 10%, 60 Hz, 3 kVA, 25 A (230 VAC ± 10%, 50 Hz, 3 kVA, 13 A in Europe)
About the Portfolio
inclusiv is a comprehensive IV compounding portfolio of integrated technology, software, and service solutions designed to support your needs for sterile compounding from the design and building of your sterile compounding environment, to the preparation and verification of your products, through the ongoing management and optimization of your pharmacy operation.
For more information, visit www.grifolsinclusiv.com

